Case Studies
Short reports showcasing quantum-informed design and AI-driven drug discovery in action.
Engineering Selectivity in a Challenging Target
Unique pharmacophore technology leads to rapid creation of novel, selective hits for DNMT1
Challenge: Off target effects limit efficacy of drugs targeting DNMT1
DNMT1 is an enzyme linked to multiple cancers. It acts on the same natural substrate as its close relatives, DNMT3a and DNMT3b. This overlap makes it difficult to design drugs that interact specifically with DNMT1 without also affecting the others.
Current drugs avoid this challenge by avoiding the enzyme’s active site and instead bind to the DNA substrate. While this indirect approach can be effective, it causes unwanted side effects.
Solution: Transition state based DNMT1 inhibitors
Even enzymes that look nearly identical can accelerate reactions in subtly different ways. Our platform uncovers these hidden distinctions—then designs molecules that exploit them to bind precisely to the intended target. The secret lies in identifying the reaction’s critical turning point: the Transition State.
How we did it - in just 2 months
From crystal structure to candidate inhibitors, our multidisciplinary team rapidly advanced drug design for DNMT1 using a uniquely integrated approach.
Result: Selective DNMT1 inhibitors
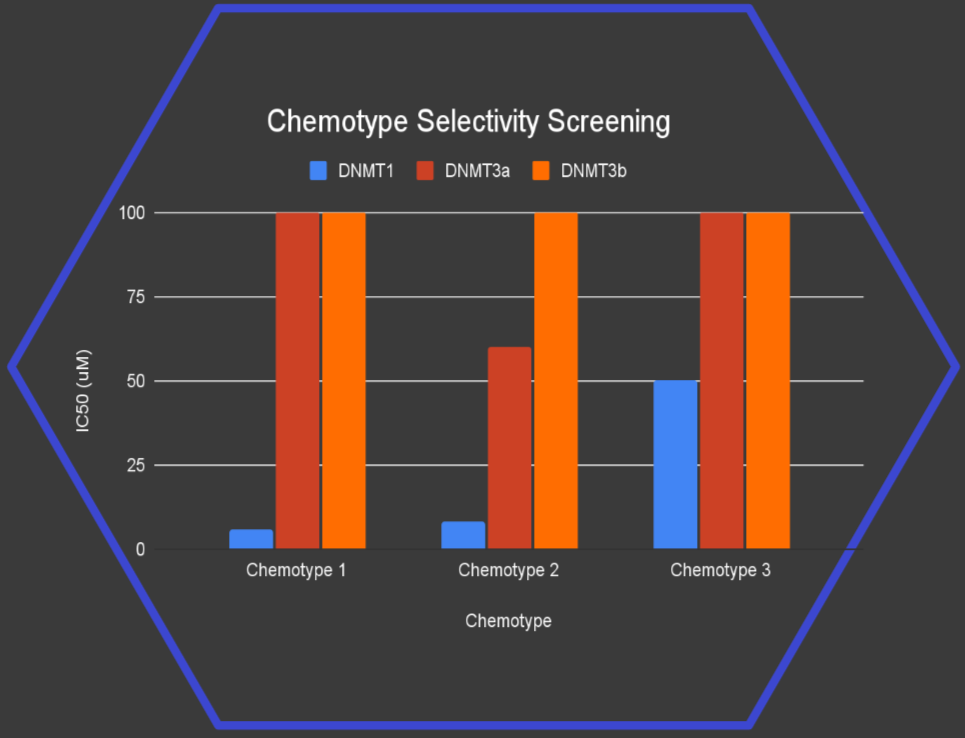
We made and tested just 40 compounds, yet found 3 novel chemotypes* active against DNMT1 with strong selectivity over DNMT3a and DNMT3b.
Notably, 2 of these chemotypes match the potency of well-established hit series reported in the scientific literature, marking a significant step forward in the search for safer, more targeted therapies.
* A chemotype is a class of molecules with related structures
Let us help you find your next advanced hit compound
Challenge: Efficient design of hits for a novel target
NOTUM is a secreted carboxylesterase that inactivates Wnt ligands by removing their palmitoleate group, which controls Wnt signaling commonly altered in cancers such as colorectal, liver, and pancreatic. Blocking NOTUM can restore Wnt driven tumor suppressive signaling and represents a promising therapeutic approach in oncology.
The clinical landscape for NOTUM inhibitors is scant and the impact of a novel inhibitor for this target would be a first-in-class therapeutic.
Rapid Generation of High Quality Hits
From crystal structure to novel inhibitors in 50 compounds
Solution: Transition State state drug design
Kuano’s Quantum Simulation identified the transition state which enabled the identification of a viable pharmacophore for de novo drug design. With the pharmacophore in hand, Our Generative AI was able to create bespoke designs for NOTUM inhibitors.
The transition state based design enables the identification of inhibitors possessing compatible charge and shape properties for engagement with the target.
Result: Production of potent and selective inhibitors
This project yielded nanomolar-potent NOTUM inhibitors with demonstrated selectivity across multiple screening panels, including same-family, pathway-specific, and broad-spectrum off-target assays. These results reflect the strengths of transition state drug design, where both potency and selectivity are integral to evaluating compounds generated by our Generative AI.
Kuano has not only found advanced hits for NOTUM but has made significant strides towards a first-in-class candidate for the treatment of bowel cancer.
Challenge: Unexplained selectivity between closely related phosphatases
In 2024, researchers publised a natural product derived series of inhibitors which were found to exhibit selectivity between phosphatases in the PTP subfamily. Most of the observed differences in binding strength could be explained by changes in amino acid sequence between the different phosphatases. However, between two of the most closely related - SHP1 and SHP2 - no differences close to the active site were present but the inhibitor bound ten times strongly to SHP2. Understanding this selectivity is key to designing inhibitors without unfavourable off target effects.
Pinpointing Selectivity Through Quantum Energy Profiling
Quantum interaction analysis decodes selectivity beyond sequence differences in SHP1/2 phosphatases
Solution: Quantum Interaction Analysis
We employed both quantum and classical methods to explain the difference between the two phosphatases. Using the Quantum Interaction component of our Quantum Lens platform we were able to provide quantitative results that explained the selectivity.
Our Quantum Lens Interaction interrogation works through Fragment Molecular Orbital analysis with a pair interaction energy decomposition analysis (PIEDA) which allowed us to:
Composite analysis of attractive and repulsive forces - A complete picture of complexation
Capture of Charge Transfer - A crucial component of ligand binding
Beyond catalytic site analysis - Identification of peripheral residues contributing to selectivity
Result: Peripheral residue energies drive selectivity
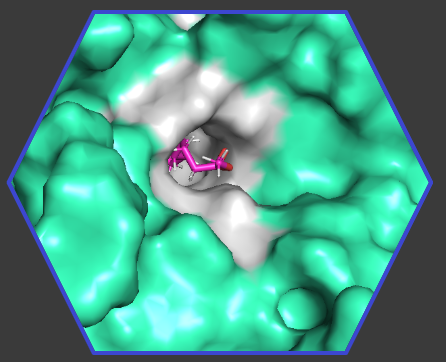
Our Quantum Lens analysis revealed that more than 50% of the binding contributions for the inhibitor came from peripheral residues (blue in the picture) beyond the active site (pink).
The differences in these residues were not picked up in the original publication but our modelling shows that comparatively unfavourable interactions far from the active site could explain the difference in binding between SHP1 and SHP2. In particular a comparison of the peripheral residue energies showed a lysine (LYS) contributing a large repulsive energy only in the SHP1 complex. This identification of an unintuitive residue interaction can enable enhanced optimisation of mildly selective compounds.
Let our quantum lens help you identify important residues
Challenge: Comprehension of covalent systems to enable Warhead Modification to enhance Selectivity
Covalent inhibitors offer a powerful therapeutic advantage by forming stable, long-lasting bonds with their targets. However, if these reactive “warheads” bind indiscriminately to off-target proteins, the result can be serious side effects and failed drug candidates.
Subtle chemical differences can modulate the reactivity, especially in the microenvironment the binding pocket. Typical experiments using glutathione (GSH) can often miss important features required for warhead selectivity.
Traditional design approaches have struggled to predict these effects. For example, the development of Ritlecitinib (a JAK3 inhibitor) hinged on switching between aryl and aliphatic acrylamide warheads — a decision driven by empirical insight rather than predictive modeling.
A Quantum Approach to Rational Covalent Inhibitor Design
Explaining the subtle determinants of target specific reactivity
Compounds with Aryl and Aliphatic warheads in complex with JAK3, with key oxygen (red) and covalent bond with the protein (green) highlighted in both structures.
Solution: Application of Kuano’s Quantum Fingerprints
Kuano uses innovative metrics to analyse inhibitors’ intrinsic reactivity and how it changes in their target enzyme.
Using quantum information approaches (based on mutualinformation and one orbital entropy) the QuantumLens platform provides a view of the systems which captures subtle energetic and electronic features missed by traditional analysis.
Mutual Information Plot of the aromatic and aliphatic systems measures a form of bond satisfaction. The red box highlights a strong bond satisfaction correlation with an oxygen on the warhead for the aliphatic system but weaker in the Aryl.
Results: Rationalisation of the difference in selectivity of the warheads
In isolation, aryl and aliphatic warheads show similar interaction patterns with GSH. Without factoring in the microenvironment, studies suggested better bond satisfaction with the aryl derivatives.
When analysing the microenvironment, mutual information plots indicate the opposite - which is consistent with the experimental results.
- Mutual information plot showed better bond satisfaction with the aliphatic system than the aryl system.
- Interaction patterns shift around a key oxygen atom in the ligand, away from the covalent bond site—subtle, but impactful.
These findings connect quantum-scale behavior to real-world inhibitor performance. More importantly, they demonstrate how computational modeling can rationalize experimental outcomes and forecast the effects of new chemical designs—including those generated by AI.
Quantum fingerprints reveal key to selectivity difference in warheads